What are Cations
Cations
The “Cation” nutrients, listed above, in your soil are very vital key to healthy soil life! The Cation Balance helps regulate the homeostasis process, or to put it simply… “That’s the crops ability to balance its total body functions. Homeostasis should be controlled and stabilized by CALCIUM! Excess MG, or NA produces a very deadly poison within the cell body, which can have a destructive effect upon the plant.
WHAT ARE CATIONS???
Cations are the positive charged nutrients in the soil. The most common cations are Calcium, Magnesium, Potash, Sodium and Hydrogen. Their balance to each other and to the other negative charged nutrients is very vital.
Following are some basic truths to consider about Cations:
- Calcium is the most important nutrient in your soil and the proper balance of it to all other nutrients is very important. Discussed in greater detail, later on in the book.
- A soil test is incomplete when the Sodium (NA) level is not included, which is common if you have been using a traditional dry fertilizer program. Sodium is the salt index to your soil WATCH IT CLOSELY!!!
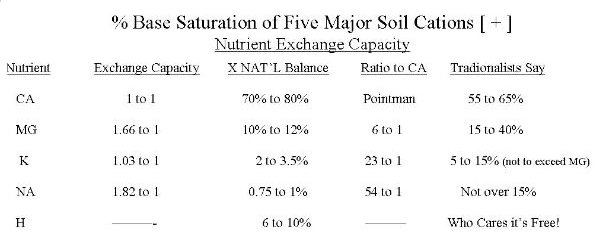
- All Cation nutrients have a HOLDING or PULLING capacity that are in relationship to calcium (CA is the base nutrient and the comparison ratios will be 1 to 1 for CA. NA the highest 1.82 to 1). This means NA has 1.82 times more pulling capacity than CA. or it has a lot more holding power.
- NOTICE: If other cation nutrients are not in proper balance to CA. then normally Hydrogen (H) will be out of balance. So watch the Hydrogen percentage level in the Cation balance instead of soil pH. If all your Cation ( + ) nutrients are in balance, both H and your soil pH is correct.
Why Are Cations So Important?
REMEMBER: The normal flow of a electric charge in nature is positive to negative! All clay and humus colloidal soil particles carry a negative charge on their surface, which attracts desirable positive charged nutrients. If in proper balance, they are loosely held in the soil solution, which allows growing plant with a (-) charged root to attract and take Cation (+) nutrients as needed.
EXAMPLE: As a plant needs calcium it removes it from the soil and it is replaced by another cation which can flow or move easily if the soil is in proper balance. This is Base Exchange at work! BUT BEWARE cations go out of balance with high usage of salty fertilization, high Mg, and heavy metal levels causing soil to tighten up with poor movement of nutrients to the growing plants. It can even get to the point, to where there are high nutrient levels in the soil and a plant be unable to get desired nutrients. KNOW YOUR SOIL!!!
CATION EXCHANGE CAPACITY (CEC) is the “ability of your soil” to absorb, hold and release nutrients or a measure of the cation holding ability of clay and humus in your soil. CEC therefore, can be measured by the percentage level of each cation in relation to 100% full base saturation. This is the critical balance we discussed earlier.
NOW REMEMBER THIS: Proper CEC balance with a healthy “soil life” which is adequate humus and biological life, helps allow the adequate release of all nutrients in proper amounts to growing crops! A healthy vigorous plant has been fed vital trace elements in proper amount to regulate its total metabolism!! Nutrients in least supply regulate growth and health, not how much NPK you can add…
An excellent example to show what can happen in the root zone when Cation balance has been destroyed with heavy use of salty fertilizers, such as 60% K20 (potassium chloride) potash base saturation can go up to 5 to 10%, sodium (salt) up to 5% and run hydrogen to zero. The Natural-Balanced pH has been destroyed by salt arc chloride (beware of soil tests that give you a false pH reading with no % base saturation balance chart). With high levels of K & NA critical nutrients like calcium, zinc, boron, and even K itself will become deficient to the plant.
REMEMBER: Soil clay particles and plant roots are both negative charged ( – ) and have a natural attraction of ( + ) cation nutrients.
Therefore, as you add nutrients to the soil, it is very important you add the right nutrients to maintain proper balance. As you add fertilizer you can help or harm your soil depending on the quality and kind of fertilizer BE CAREFUL WHAT YOU ADD!!
5 Major Soil Cations:
POTASSIUM
(K) = Expressed in ppm x 2.4 = Lbs. available K/acre. Natural Balance levels of K vary according to CEC of soil and graduate up. Rates above this can cause problems in the soil, if achieved thru commercial fertilizers. Also, it is not economical to maintain a higher level!
MAGNESIUM
(MG) = Expressed in ppm x 2 = Lbs. available MG f acre. Excessive MG is found in many areas of the USA because of recommended dolomitic limestone and fertilizer programs. If most soils were put on a good natural biological program, they would not need any MG for years and years…..
The biggest lesson to learn on MG is how to bring the MG level down to a desirable level, and to do it economically. Excellent practices would be turning under green manure crops, or applying a stabilized biological product.
CALCIUM
(CA) = Expressed in ppm x 2 = Lbs. available CA/acre. SPECIAL NOTE: If percent saturation of cations is out of balance, or toxic residues exits (aluminum oxide, iron oxide, NA. or mg). The above available calcium may not be exchangeable to the plant! Remember, nutrient balances are much more important to watch than nutrient levels.
SODIUM
(NA) = Expressed in ppm x 2 = Lbs. available NA/acre. Nearly all Universities and Independent soil testing labs do not run test on sodium! Mr. Farmer, include NA in your soil analysis, and learn how to apply its percent saturation into a well balanced cation balance. See earlier discussion of recommended levels of sodium.
SULFUR
(S) = Expressed in ppm x 2 = Lbs. available S/acre. (1 lb. sulfur = 3lbs. sulphate). Adequate S levels in your soil should be 10-18 ppm or 20-36 Lbs. S/acre. EXAMPLE – In a healthy 3 % OM soil 60# S would be a very favorable N to S ratio (2 to 1).
Sulfur levels can be increased to 15-25 ppm on medium to high CEC soils, while purging of heavy metals. More sulfur is needed to help remove undesirable metals mentioned earlier as MG.
Sulfur is a vital nutrient in our Natural BALANCE PROGRAM that has been neglected in modern agriculture. A healthy, living soil supplies adequate sulfur to crops thru decomposition of crop residue, manures and biological activity. The problem with modern agriculture is that fertilizer products like NH 3 82 %, potassium chloride, DAP 18-46-0, ammonium polyphosphates, 10-34-0 deplete humus levels, suppress beneficial bacteria, and create nutrient imbalance. Then the soil does not release natural sulphate into the soil solution for crop intake.
Let’s Analyze Each “Cation Nutrient”
CALCIUM (Ca) The KING of all nutrients! Proper amounts of Ca (70% and up) makes soil workable and well flocculated, plus it has a good air-water relationship. Air and water are two “free” elements of nature that are very vital to plant growth and production. Any nutrient that aids in a good air-water relationship is a priceless asset and Ca is the prince of nutrients in maintaining this vital balance.
Proper levels of nutrients, organic matter, water and air makes soil productive.
Calcium helps create a healthy environment for your plants plus it is the carrier (or trucker) of all other nutrients to the plant. As Calcium content in the plant drops so can the protein, energy level and minerals of the plant. Calcium stimulates growth of “soil life”, including nitrogen-fixing bacteria.
Calcium is sweet alkaline earth, and the opposite of true acids (Na and K) and concentrated salts products like ammonia nitrate, and potassium chloride (0-0-60). Any product that you use that removes calcium from the soil can be EXPENSIVE AND DANGEROUS!!! The following products require Ca, or calcium carbonate equivalent per ton applied – anhydrous ammonia-2960#, DAP-1550#, ammonium sulphate-2200#, etc; also, 0-0-60 K removes 23# Ca per 100# K20 applied WHAT A WASTE—. Please don’t waste our “Natural Neutralizer” Ca by using harsh fertilizers.
Did you know the following facts about CALCIUM?
- The lower the ph (Ca level) in the soil the greater the leaching loss of K and NH4.
- The percentage of CEC saturated with Ca is more important than the total amount of Ca in the soil (high % Ca can stop toxic aluminum, iron and Na conditions).
- Studies showed decrease root development was not related to ph, but to low levels of exchangeable Ca and high levels of aluminum.
- Soil Scientist (world-wide), which are independent of petroleum-agronomy influences, normally agree to a Ca-MG ratio of about 7 to 1 for an ideal soil.
- REMEMBER Ca IS AS IMPORTANT to humans as plants.. Did you know calcium is only absorbed in the small intestines with adequate vitamin D, but excessive fat, oxalates and in-organic phosphates inhibit calcium absorption. Less than 15% of the Ca in homogenized milk is used because of the heating process which kills beneficial enzymes and makes Ca unavailable. What a shame!!! Best sources of Ca are a raw dairy products, calcium lactate, almonds, whole grain seed and dark green leafy vegetables. EAT RIGHT’N LIVE.
- Study after study shows Ca at the optimum level will decrease disease in most plants. With lower percent saturation of Ca combined with high levels of MG and K; showed more disease problems, such as seedling rots, soft rot in stored tubers, and various root and stalk rots.
- High content calcium hay, haylage, or forage crops play a vital role in healthy, vigorous animals. Ca is normally associated with healthy soil full of soil life.
Plant available calcium determines the uptake of all other nutrients into the plant.
Why does calcium make so much difference? How does it release other plant nutrients in the soil to give you, in effect, a free bonus of fertilizer? Here are some of the important facts that the soil scientists have been discovering.
Soils generally contain large amounts of aluminum and iron. In acid soils, aluminum and iron combine chemically with soil phosphorus and tie it up in unavailable forms. But when you lime the acid soil, this tie-up of phosphorus is prevented from taking place, and phosphorus already tied up is released slowly!
Calcium works the other way, too, cutting down excesses of some minerals. Acid soils containing too much soluble manganese are as harmful as soils containing too little. Growth is slowed; some plants may even be stunted. As the soil gets more acid, more of these minerals become soluble. Add calcium, and it ties up enough of them so they are no longer toxic to plants.
When you use calcium on acid soils, you save potash, too. When plants can’t get enough calcium, they take up more potassium from the soil. But when there’s plenty of calcium, plants will use more of these nutrients and they’ll not use excessive amounts of potassium. So, on a properly limed soil you can produce good crops and not exhaust the soil potassium supply as rapidly.
Yellow alfalfa is yellow partly because it was not getting enough nitrogen. Plants get nitrogen from three sources: From the air, from soil organic matter, and from fertilizers. And in every case, except with some forms of fertilizer, the nitrogen must be worked on by soil microbes before plants can use it.
Using calcium on acid soil helps these microbes which take nitrogen from the air. The present theory of how soil acidity effects microbes is that the lime neutralizes the acids produced by the microbes while they are converting the nitrogen to available forms.
And this affects more than just nitrogen. Before your crops can use many of the plant nutrients in the organic matter that is returned to the soil, that organic matter must pass through a whole assembly line of soil microbes.
But before this assembly line can operate, there must be enough calcium and magnesium for these microbes to thrive and multiply.
Too, the microbes that make streptomycin, aureomycin, penicillin, and other antibiotics which kill or make harmless the disease-producing microbes in the soil, must have calcium before they can really thrive and work.
So, calcium indirectly helps control plant diseases because it peps up these antibiotic-producing microbes.
Why is Available Calcium So Important?
Calcium is the most important fertilizer element
Is a direct nutrient to growing plants
Neutralizes soil acidity
Improves activity of favorable soil bacteria and the decomposition of organic materials
Promotes root development
Improves soil structure
Promotes nitrogen fixation by legumes
Improves the efficiency of fertilizers and chemicals
Increases water penetration and water holding capacity
MAGNESIUM
Its percent of base saturation is second place to calcium. But nature REQUIRES A MINIMUM of 6 to 1 ratio to calcium (example Ca 72% to MG 12%). Then MG will perform its duty in photosynthesis and protein formations. Over-saturation will hinder these vital functions…
OPTIMUM Ca to MG ratio is 7 to 1, which promotes soil life and adequate nutrient levels for growing crops. We recommend a percent base saturation of 10 to 12% MG. Little concern should be given to MG deficient soil because of its high holding-pulling capacity. MG deficient soil would normally be on sandy soils with less than 6% base saturation and loam-clay soils at less than 4%! Traditional fertilizer/agronomy personnel keep calling for MG in the form of dolomitic lime, Sul-po-mg, MG sulfate, etc. WHAT IS THE PROBLEM??? Let us get to the bottom of the problem and not treat the symptom of the problem as most educated traditional personnel do!
What can create a MG deficiency? Potassium, Sodium, Boron, and an unbalanced pH with heavy metals such as aluminum, and iron. We can see real quickly their own recommended fertility program has created the problem. First 0-0-60 KCL with a salt index of 116 applied to the soil is a “bomb of K and chlorine” that unloads undesirable sodium. High sodium releases toxic levels of aluminum and iron, this toxic combination causes a deficiency of MG, Ca, and K.
Second: They recommend DAP (18-46-0), or NH3 to add to the MG deficiency. THE AMMONIA bomb is dropped and soil pH can jump to nearly 10 for a short period of time. Undesirable high levels of salts are released and “soil life” is STOPPED! MG and Ca both can become deficient.
THE BAD NEWS OF MAGNESIUM
- If MG is out of balance, it can become a toxic poison to soil, calcium and plant life.
- Excess MG to Ca forms a poisonous condition in the nucleus of plant (man) cells, affecting its health and the continuing life of the plant. Nature often strikes the plant with diseases (fungi) to destroy the plant and simultaneously nature calls for other plants to take its place–commonly called WEEDS AND GRASSES!!
- With excess MG or Na you are on your way to producing concrete out of your soil. It resists water, it has less air (oxygen), less soil life, more weeds (acid soil), more insects (less plant sugar), and more plant diseases (soil acid). Weeds and grasses are nature’s soil indicator of danger… You have an imbalance in the soil. You need a new fertilizer program, not HERBICIDES, INSECTICIDES, FUNGICIDES OR SUICIDE.
- Excess MG means deficiency of N, P, K, and Ca. Yes, the big four go deficient! Only MG with combination of Na, AI, and iron can make concrete out of soil, it destroys the air-water ratio necessary for soil life. SOIL EFFICIENCY IS DESTROYED. These MG-AL-NA combinations are toxic to animals too. MG takes the place of Ca in plant cells which produce a poor quality crop.
- Excess MG to Ca permits crop residues to decay into toxic alcohol, which kills or suppress bacteria, it then forms a formaldehyde, which stops crop residue decay (this means disease carry over).
- Excess MG will not allow CA and K to move into plant cells (plant absorption is slowed down considerably).
- Excess MG to Ca causes your soil to release soil nitrogen back to the atmosphere (these are in the form of gaseous nitrogen oxides). WHAT A WASTE.
POTASH
It makes up only 2-3% of the CEC, but potash is the #1 dry fertilizer sold to AMERICAN farmers! Why?? Has the American farmers been sold excessive K?
Yes, and in a form undesirable to our soil. Who has made the rules of mandatory nutrient required to produce 150bu corn, 45bu soybeans, 60 wheat, etc.?
Guidelines and recommendations come from the National Fertilizer Institute, thru Land-grant Universities, Cooperating Co-ops, etc.
The experts, who tell us, our crops require “specific” poundage of NPK, forgot to tell us there are other sources, than to purchase NPK from the local fertilizer dealer.
EXAMPLE: To raise 150bu corn (grain) it removes 135# N, 53# P, and 40# K. They forgot to tell us that our healthy silt loam soil can have a K reserve of over 35.000# (300 to 400 available K in balance soil). Plus earthworms can add K to your soil fast, earthworm castings have 11 times more K than soil particles and organic residues that run through them. WHAT A WASTE OF MONEY TO BUY UN-NEEDED 0-0-60. K is a necessary nutrient which aids in photosynthesis, protein formations. And size n’ quality of fruit. K is very important (20,000 to 35.000# per acre.)
The #1 potash product is potassium chloride (KCL) or muriate of potash. Muriate of potash can be red or white. Most kalium is white muriate of potash. Muriate is a salt of hydrochloric acid. Farmers on natural-organic programs should never use killers of “soil life”.
Chemically K reacts in soil to release sodium (salt). High usage of KCL adds too much NA, which prunes plant roots and will not allow water and nutrients to flow freely to the plants. BEWARE it is approximately 47% chlorine and it can become a KILLER TO SOIL LIFE, animals and people. It is quickly leached from the soil, or is locked up in fixed non-exchangeable K with less than 20% actually used by the crops in many soil conditions.
MURIATE OF POTASH 0-0-60 K, 20 is actually only (approx.) 51.7 % K and 47% Chlorine. Chlorine is a gas that kills life! Research indicates Chlorine is a wild cat element and is seldom broken down, but continues to be tax c the soil and water.
LET’S LOOK AT CHLORINE – KCL or Potassium chloride, or more commonly called muriate of potash is 47% chlorine. Therefore, a 100# (actual K) application will add 42 to 45 ppm of chlorine to your soil, which is about 12 times the chlorine it takes to sterilize water!
Potassium acts like Sodium
K acts like Na (two true acids). They are fast acting, and there is no need of trying to build a reserve of K through commercial fertilizers. It will brake you financially, so don’t play their game of recommended soil nutrient levels, you can’t win at their game, so do it NATURE’S WAY.
Some closing thoughts on potassium:
- A very small percentage of broadcast applied K will become exchangeable during the growing season, or even show up on a soil test. WHY? Most commercial K is 100% water soluable on soil contact; therefore making it is highly leachable (up to 75%) on sandy soil and most of the remaining K is fixed in the soil due to unbalanced conditions (even the CI makes K unavailable). Most commercial K is broadcast applied in early spring and can move out of the soil quickly with springs rains. SAD TO SAY – A plants’ need for K starts out in small amounts in a young growing plant. In corn, less than 5% of total K needed, is used from germination to approximately 38 days after emergence (when tassel begins to develop).
- Most of the K that a plant will use is obtained by diffusion. That means the magnetic pull of the root draws the K to itself as needed (K must be within % ” of the root to be exchangeable). This is why direct seed placement and foliar sprays can be very profitable at very low rates. Well flocculated, aerated soil has a much higher uptake of K by roots. Adequate moisture is required for K movement.
SODIUM
SODIUM THE OTHER TRUE ACID LIKE K!!!
Crop tissue analysis ranks sodium number 12 in amount, plus it is not essential to plant tissue. The fertilizer empire promoters say, “Sodium is of no consequence and they don’t test sodium levels unless specifically requested”.
SO WHAT’S ALL THE FUSS ABOUT SODIUM (Na). Excessive Na is an enemy to soil life, animal and man. Someone says, “There is no Na listed on our fertilizer analysis, so this commercial fertilizer can’t add Na”. The truth is “yes it can”. K activates Na. Even though there is up to 25,000# of Na per acre in our soil it is no problem, if you farm nature’s way…But if you add large amounts of K you are asking for trouble, Na is released into the soil solution and a number of harmful things can happen. (Every pound of K you add to your soil can add up to 2% to 3 times of Na to the soil solution and soil nutrient complex).
- Too much Na or MG can kill “soil life”. EXAMPLE: desert land is sterile because of Na. An early method to control Johnson grass was by killing the soil with the use of NaC!!! Sodium chloride is a natural killer, so is 0-0-60 if you use high rates (salt index 116.3).
- Na produces water stress on a growing crop and actually has the pulling power to hold water from roots. The soil can have adequate moisture and the crop actually starve for water.
- Na has the strongest exchange capacity of any cation (1.82 times more pulling power than Ca). A small amount of Na WILL TAKE CONTROL IN YOUR SOIL.
- Any commercial fertilizer, with a salt index above 70 should be used with caution. Also, some man-made products like anhydrous ammonia, or any ammoniated phosphates may show a low salt index but in the soil it immediately creates a very alkaline (salty) reaction…A strong salty solvent solution is formed, which is deadly to plant roots and soil life. FIGHT SODIUM WITH NATURAL SULFUR… Soluble Calcium, Humic Acids, and a good sugar enzyme-biological program are all good methods to restore Na to a desired level. Most excessive Na levels can be brought into balance within 1 to 2 years on most soil types.
- To get a true picture of the Na balance in your soil ask for Na to be included in your cation base saturation test, PLUS a water soluable test on Na can be very helpful. Na (salt) should NEVER exceed Sulfur on the water soluable test.
HYDROGEN
HYDROGEN THE FREE-EXCHANGE NUTRIENT, it takes last place at the cation table.
HYDROGEN only takes an empty place at the table, if it is there! Soil that has no H (or no acid) has nutrient limitations. Acid in soil solution is what allows most nutrients to become exchangeable to plant roots. Most soil microbes thrive on these slightly acid conditions. So, watch your soil audit closely and monitor H percentage base saturation. Proper soil pH will normally be 6.4 to 6.8.
OPTIMUM NATURAL LEVEL OF HYDROGEN is 6 to 10%. If the soil has over 15% H, it becomes too acid (low pH is normal). If hydrogen is at zero, this tells you the other cations are out of balance, or over saturation has occurred with one or more cations. So, hydrogen should be watched very closely… Your “soil life” depends on it.
NOTICE: This is why keeping a “proper balance” of nutrients is much more important than dumping fertilizer by the ton. A few pounds of an active, over-saturated nutrient, like K, Na or MG affects the soil much more than hundreds of pounds of calcium. Man’s unnatural “true acids” and “acid-salt” fertilizers are destroying our soil balance. Crop diseases, weeds and insects come our way as a warning that our soil is in DANGER!
FACTS TO CONSIDER ABOUT SULFUR…..
- The sulfur/nitrogen balance is very important to a plants metabolism and energy level. In the photosynthesis process sugars are combined with natural phosphorus, nitrogen, and sulfur to complete the PROTEIN ENERGY/AMINO ACID COMPLEX. Their balance to each other is very vital, and over saturation of N and deficiency of P or S results in poor quality crops. Actual plant tissue ratio levels (the N to S ratio vary from 15 to 1 in young corn to 12 to 1 in silk stage). Soybeans will run fairly consistent. Balance of sulfur in the sugar complex allows the plant to stand more stress from weather, disease, etc., EXAMPLE – PLANTS HAVE A HIGHER FREEZING TEMPERATURE WITH PROPER S balance (more resistance to frost in early spring).
- Proper S levels build higher & better enzyme complex systems.
- Did you know that proper S to N balance increases nitrogen efficiency by a plant (and so can pure corn syrup)
- Sulfur increases protein in grain & grasses, and controls nitrate build up (toxicities) within corn and forage crops.
- Sulfur can be used as a valuable tool to lower pH of alkaline soils (pH 7.2 & higher); therefore increasing the availability of other key nutrients such as Phosphorus, Manganese, Boron, Copper, Zinc, & yes, Nitrogen! It also, can be used to control Sodium, Magnesium, Calcium & salt build-up in problem soils. Yes, Sulfur can be the “KEY NUTRIENT” to improve the physical condition of your soil.
Summary of the Five Cations
[CA, MG, K, NA, & H)
On any soil audit, or soil test the actual pounds of each nutrient available is not nearly as vital as the proper balance between each other. Specifically watch their balance to Ca. Remember this important fact-high levels of a nutrient on a soil test means nothing to a crop fighting a war against severe imbalances, which cause nutrients to become fixed in the soil or even toxic to the plant. Therefore, watch your “balance of nutrients” and use safe, common sense farm practices that promote balances and soil life.